HOME >> NEWS >> Industry Articles
In the evolution and application of sulfide ore collectors, xanthates and their derivatives play a significant role. Through in-depth research on their chemical properties and performance in the flotation of different ores, we have continuously improved ore recovery rates and mineral processing efficiency.
◆ Properties and Applications of Xanthates
Xanthic acid (R-O-CSSH) itself is unstable, appearing as a colorless or yellow oily liquid. It is slightly soluble or poorly soluble in water and may cause violent explosions during decomposition. However, its alkali metal salts exhibit high stability. For example, sodium salts are highly deliquescent and form dihydrates, while potassium salts do not deliquesce. These salts are easily soluble in water, alcohol, and acetone.
In the flotation of complex polymetallic sulfide ores, xanthates demonstrate remarkable collecting performance. Generally, the longer the carbon chain in the molecule, the stronger the collecting ability. However, it is worth noting that xanthates with shorter carbon chains exhibit higher selectivity, while those with longer carbon chains have poorer selectivity. For instance, sodium ethyl xanthate offers the best selectivity, and sodium isopropyl xanthate is widely used abroad due to its low production cost, strong collecting power, and good selectivity. Sodium isobutyl xanthate exhibits even better collecting power. Potassium amyl xanthate has the strongest collecting power but relatively poorer selectivity.
To prevent the decomposition of xanthates in highly acidic pulp, their dosing concentration is typically controlled at 10–20%, with a general dosage of 23–90 grams per ton of ore.
◆ Xanthate Esters and Trithiocarbonates
Xanthate esters are stable and suitable for vacuum distillation. They are oily substances at room temperature and insoluble in water. They are often added to ball mills and serve as effective collectors for copper minerals. Even in lime-added pulp, they can act as good collectors for zinc. Notably, these substances do not collect pyrite and often enhance the recovery rates of gold and silver in sulfide ores.
Trithiocarbonates are compounds formed by replacing the oxygen in xanthate molecules with sulfur. They are also effective collectors for sulfide ores. Although their production costs are higher than those of corresponding xanthates, they began to be used in the flotation industry after patents filed in countries like Belgium in 1982 demonstrated that trithiocarbonates could yield good benefits in molybdenite flotation.
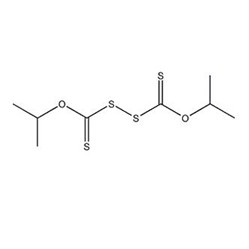
◆ Thiocarbamates and Aerofloats
Thiocarbamates are compounds formed by replacing the oxygen in xanthate molecules with nitrogen. They are excellent substitutes for xanthates, offering superior selectivity and requiring lower dosages (5–20 grams per ton). Additionally, they are less prone to decomposition in acidic pulp.
Aerofloats, as important collectors for sulfide ores, rank second only to xanthates. They are primarily divided into cresylic aerofloats and alcoholic aerofloats. In alkaline pulp, cresylic aerofloats do not float pyrite, pyrrhotite, or unactivated sphalerite. Meanwhile, they remain stable in acidic pulp, allowing pyrite to be floated simultaneously. It is worth noting that the ammonium salt of cresylic aerofloat has weak foaming properties. Alcoholic aerofloats, such as sodium aerofloat, #208, #211, and #238 aerofloats, also do not float pyrite in alkaline pulp. Moreover, they exhibit weak floatability for galena, making them ideal selective collectors in the separation process of copper and lead.
 关注微信
关注微信